
Company Profile
As a national high-tech enterprise, Shenzhen Maide Biotechnology Co., Ltd. specializes in the R&D, production and sales of high-value medical supplies, with products widely applied in key clinical departments including Interventional Department, Anesthesiology & ICU, Obstetrics & Gynecology Department, Urology Department, Orthopaedics Department, and Medical Imaging Department.
Our core product portfolio covers percutaneous transhepatic drainage devices, disposable pressure transducers, angiographic accessories, tracheostomy intubation kits, balloon uterine stents, and disposable closed suction catheters—catering to diagnosis, treatment, and nursing needs across clinical scenarios.
Maide focuses on developing and manufacturing high-precision medical molds, including those for PC, ABS, PVC, PP, PE medical plastics as well as silicone and blow-molding processes. We also provide comprehensive ODM and OEM services to meet partners’ customized requirements for product specifications, packaging, and branding.
Our products are not only sold nationwide in China but also exported to global markets. Adhering to a scientific development model integrating production, education and research, we strictly follow international medical device quality management norms in all links (from raw material inspection to finished product testing) and are in the process of advancing relevant certification applications. We have earned wide recognition from customers through superior product quality and trustworthy services.
How We Innovate
Experts + Real Needs
We work closely with doctors and nurses to fix the actual challenges they face in daily workflows.
Full Process Control
We established an integrated closed-loop covering R&D, mold design, intelligent production, testing and delivery.
Results-Driven
With patented technology and university partnerships, we turn ideas into practical solutions faster.
Why Us
Compliant Production & Advanced Facilities
We strictly produce in line with ISO13485:2016 standards and relevant regulations, with a self-built 1,300㎡ Class 10,000 clean workshop. It is equipped with constant temperature and humidity systems, purified water systems, ethylene oxide sterilization systems, and supporting physical, chemical, microbial testing laboratories—all tailored to ensure the high quality of our core interventional products (e.g., percutaneous transhepatic drainage devices).
High-Quality Control
We have a professional QC team and 3-stage inspection (raw material testing, in-production sampling, finished product verification) to focus on our flagship interventional products, ensuring each percutaneous transhepatic drainage device and accessory meets strict clinical safety standards and ISO, CE certifications, along with other consumables like tracheostomy kits.
Fast Delivery Guarantee
We have flexible production shifts and a responsive scheduling team, with priority support for orders of our core interventional products. Even for urgent orders of percutaneous transhepatic drainage devices, we can shorten the production cycle by 20% while maintaining quality, helping partners quickly seize interventional market opportunities.
Professional R&D Capability
Our R&D team has 5+ years of medical consumable experience, with a key focus on interventional product innovation. Cooperating closely with clinical experts, we continuously optimize percutaneous transhepatic drainage device performance, along with upgrading anti-kinking pressure extension tubes for better interventional procedure support.
Customized OEM Service
We have a dedicated OEM team providing one-stop customization, with specialized solutions for interventional products—adjusting specifications of percutaneous transhepatic drainage devices (size, material), designing exclusive packaging, adding brand logos—to fully match partners’ interventional market positioning and clinical needs.
Comprehensive After-Sales Support
Our professional after-sales team offers 7×24-hour technical consultation and problem-solving. For product usage guidance or quality feedback, we respond within 4 hours to ensure partners’ smooth operations.

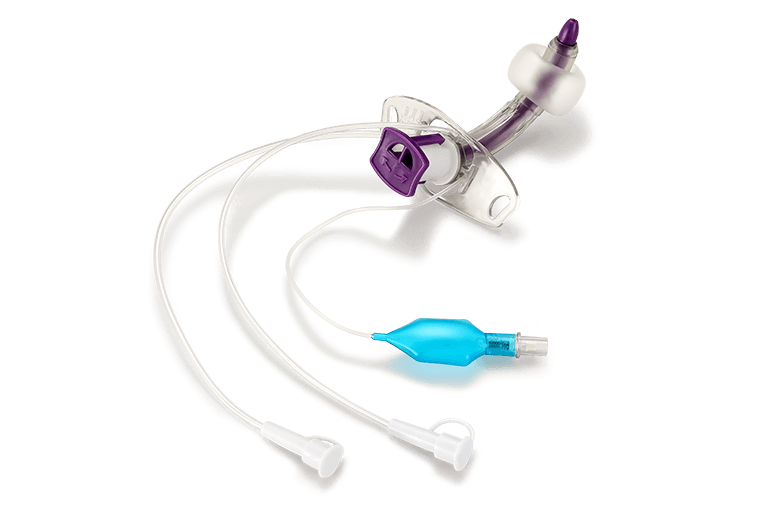 Anesthesiology ICU Products
Anesthesiology ICU Products Interventional Products
Interventional Products Medical Image Products
Medical Image Products Obstetrics & Gynecology Products
Obstetrics & Gynecology Products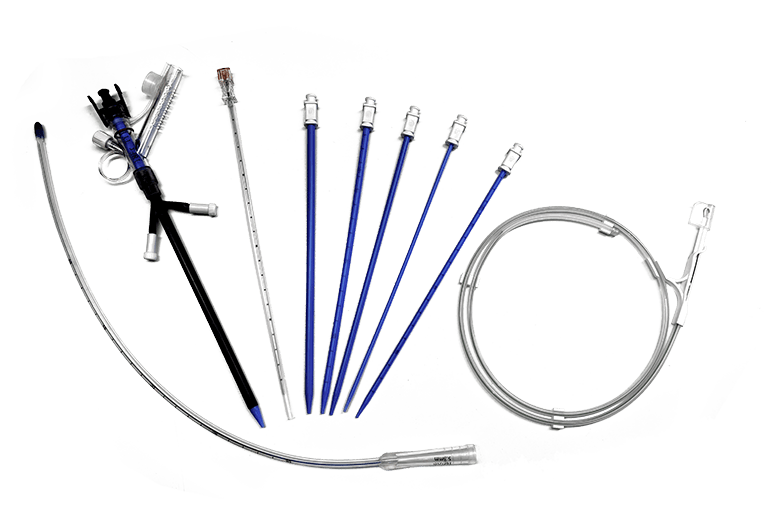 Urology Products
Urology Products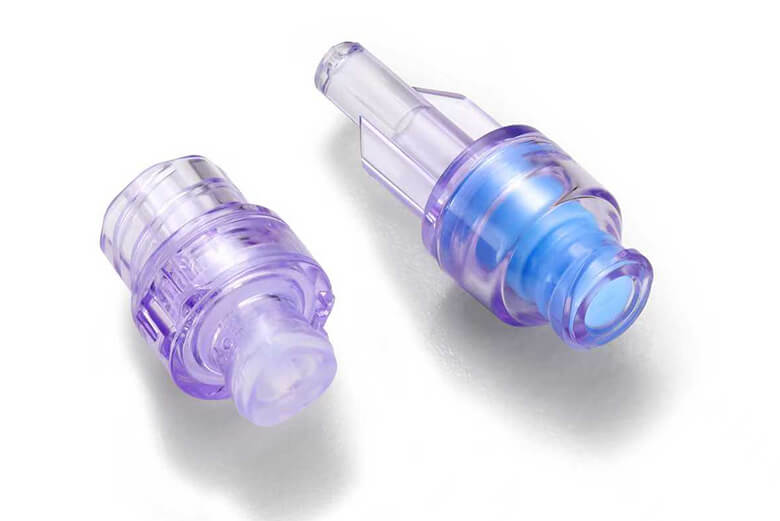 OEM Products
OEM Products













